Dyes That Don’t Pollute: The Rise Of Ionic Liquids In Sustainable Fashion
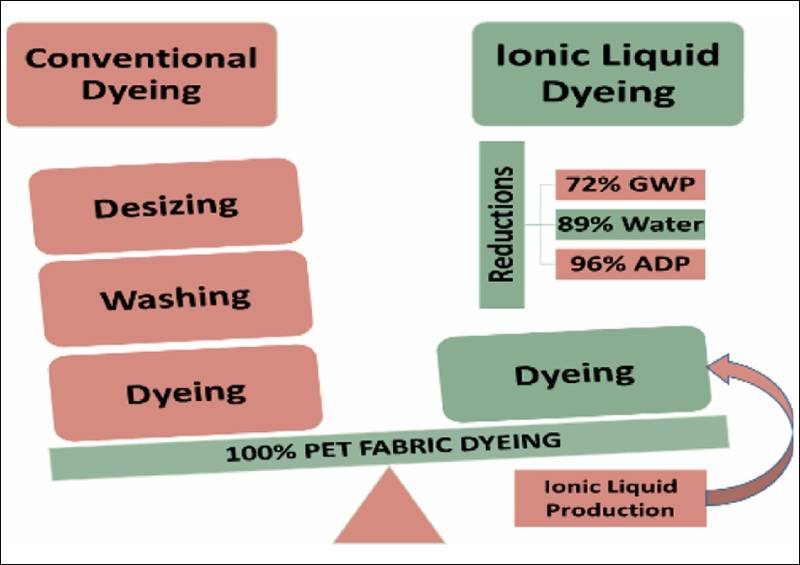
The fashion industry dazzles with its colours, textures and trends, but behind the curtain, it is one of the biggest polluters in the world. One of its most environmentally damaging practices is dyeing fabric, particularly polyester, which dominates the market. Traditional polyester dyeing consumes vast amounts of water, chemicals and energy, requiring high heat (around 130°C) and generating wastewater rich in toxic residues. With 40 billion cubic metres of water used annually to produce 60 billion kilogrammes of fabric, the scale of this impact is staggering. However, an innovative alternative is emerging that could change everything: ionic liquids.
Ionic liquids (ILs) are salts that remain liquid at room temperature and are celebrated for being “green solvents”—they don’t evaporate, are reusable and can be custom-designed for specific tasks. Researchers have recently explored the potential of ILs like 1-butyl-3-methylimidazolium chloride ([Bmim]Cl) in textile dyeing. Unlike conventional methods, IL dyeing can be done at just 95°C, dramatically reducing energy consumption. But this isn’t just a lab-based fantasy. A new study has, for the first time, performed a comprehensive industrial-scale life cycle assessment (LCA) comparing conventional and IL-based dyeing methods.
The results are striking. Dyeing 1 kg of polyester fabric using ILs reduced the global warming potential by 71.88%, acidification by 53.72%, water consumption by 88.93%, and toxicity-related impacts by up to 92%. These benefits stem from simplifying the process into a single dyeing step, eliminating harsh chemicals, and lowering the dyeing temperature. Notably, the method still achieves excellent colour quality and fastness, matching or even exceeding traditional results.
 However, there’s a caveat: the production of ILs is not entirely benign. Synthesizing [Bmim]Cl requires multiple chemical precursors, including N-methylimidazole and glyoxal, which themselves contribute to environmental impacts through energy use and emissions. Even so, the overall life cycle impact of IL dyeing remains far lower than that of the conventional process, making it a promising green alternative.
However, there’s a caveat: the production of ILs is not entirely benign. Synthesizing [Bmim]Cl requires multiple chemical precursors, including N-methylimidazole and glyoxal, which themselves contribute to environmental impacts through energy use and emissions. Even so, the overall life cycle impact of IL dyeing remains far lower than that of the conventional process, making it a promising green alternative.
What makes ILs even more appealing is their potential for recovery and reuse. Using a salt-based aqueous extraction method, up to 90% of the IL used in dyeing can be reclaimed. This recycling reduces freshwater aquatic ecotoxicity by 70%, water use by 23%, and carbon emissions by over 11%. While recovery adds complexity, the environmental and economic advantages are substantial, especially considering that ILs are currently five to twenty times more expensive than traditional solvents.
The study also examined how IL concentration affects sustainability. Reducing the IL dose from 5 g/L to 2 g/L resulted in improved environmental outcomes across all categories, whereas increasing it to 15 g/L worsened the impacts. This sensitivity analysis underscores the importance of optimization in scaling the process sustainably.
In conclusion, IL-based dyeing represents a breakthrough opportunity for the fashion industry to reduce its environmental footprint dramatically. While challenges remain such as high production costs, potential toxicity and industrial scalability, the benefits are undeniable. With further innovation in IL synthesis, recovery technologies, and application across various fabric types, ionic liquids could transform textile dyeing from one of the dirtiest processes to one of the cleanest.
As the world demands greener fashion, ionic liquids offer a vibrant, sustainable path forward proving that true style does not have to cost the earth.














